 Dear Mr./Ms.,
Dear Mr./Ms.,
Sanyou Biopharmaceuticals focuses on providing integrated solution for therapeutic antibody discovery and development and offers all sorts of services for therapeutic antibody R&D.
In terms of industrial production cell line development for antibody, the platform is cGMP compliant, and it only takes about 3.5 months to obtain stable single-clone and high-quality cell lines with high expression up to 8-10 g/L. We have experiences of construction and characterization of nearly 20 industrial cell lines.
ADVANTAGES
1) High Expression: Up to 8-10 g/L.
2) High Stability: 2 rounds of stability assessment were performed to fully assess cell line stability in terms of cell line passaging stability, cell growth stability, antibody expression stability, gene sequence identity and gene copy numbers.
3) High Quality: 17 cell library quality control indicators, 11 antibody product quality control indicators, and 9 cell stability quality control indicators monitor the cloning screening process and guarantee high quality cell lines.
4) cGMP Specifications: The cell bank construction complies with cGMP standards. The monoclonal origin of the cell line meets the regulatory requirements and the image data is complete. All documentation records are clear and traceable to ensure that the source of seed cells is clear, the cell line transmission records are clear, and the cell bank construction and validation records are clear.
Advance your mAbs to scale-production.
SERVICE
| Service |
Input |
Time |
Output |
| Primary Screening Clone Construction |
Seq. of antibody
|
5~6 weeks |
3~5 selected highly expressing primary clones |
| Monoclonal Screening and Library Construction |
10-14 weeks |
1) THREE highly expressing monoclonal cell lines, ONE of which is used to construct the master cell bank and working cell bank
2) Reports and records of cell line construction
3) Reports and records of cell bank construction
|
| Complete validation and stability analysis of the cell banks |
16-20 weeks
|
1) Reports of cell bank validation
2) Reports of stability study
|
EXAMPLE
The monoclonal cell line was inoculated into the culture medium for shake flask Fed-batch culture, and regular samples were taken to detect the metabolic data, such as live cell density, cell viability, titer, and concentrations of lactate and ammonium ion (Fig.1). The cell density was up to 15.9×10^6 cells/mL, and the cell viability was maintained at 81.6% after 19 days of culture. The antibody expression was up to 8.8 g/L, and lactate and ammonium ion concentrations were always maintained at relatively low levels (lactate <2 g/L and ammonium ion <10 mM).
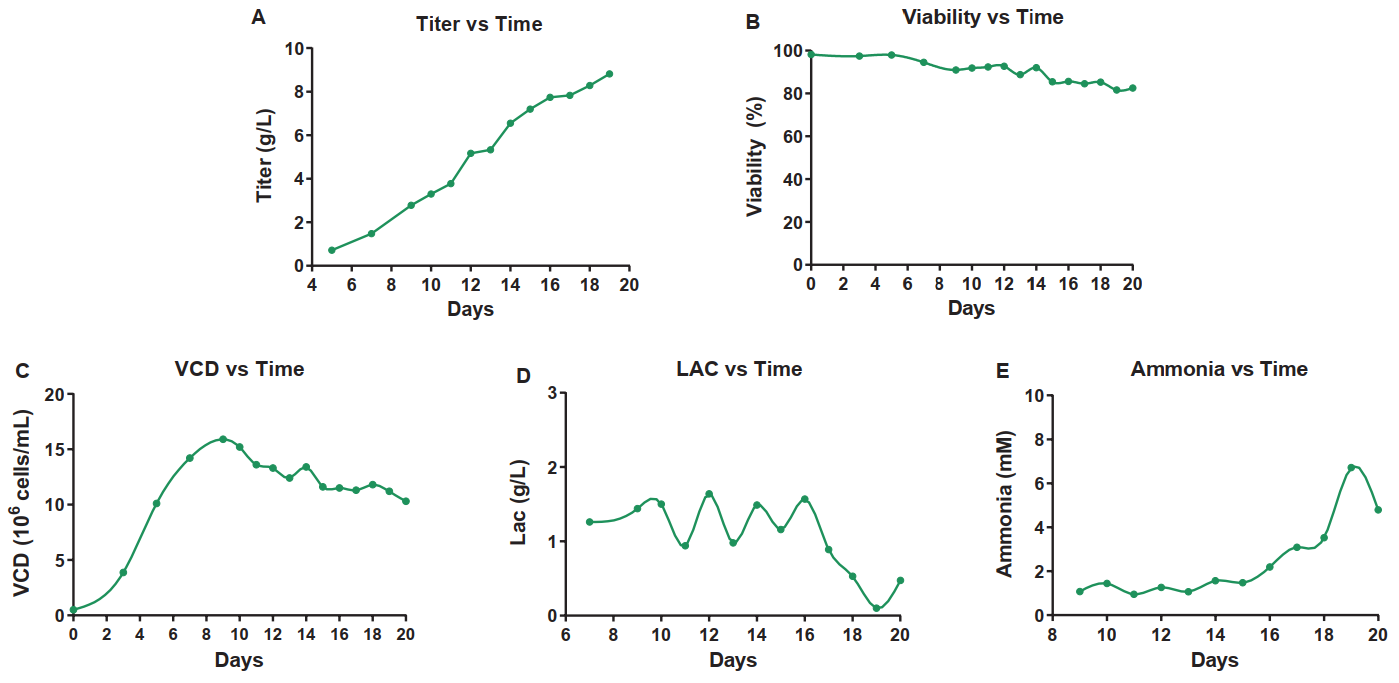
Fig. 1 Shake Flask Fed-batch Culture of Monoclonal Cell Line
Do you need assistance with mAb scale-production?
ABOUT US
Sanyou Biopharmaceuticals is a leading international High-tech biotechnology company focused on research, development and providing integrated solutions for innovative antibody drug developments.
Sanyou Bio is committed to building world-leading antibody drug discovery and development platforms of high-quality, high-throughput, integrated R&D and value transformation. Sanyou Bio is dedicated in constructing a business ecosystem for therapeutic, R&D and diagnostic products and services, and collaborating with global biopharmaceutical, diagnostic, R&D companies to open a new era for the diagnosis and treatment of human diseases.
Since the founding in the year of 2015, Sanyou Bio has been growing rapidly. As of May 2021, Sanyou Bio has a professional team of more than 160 employees, of which more than 70% hold a PhD or master degree, and this team is deeply experienced in R&D and industrialization of innovative drugs. Sanyou Bio has established an integrated R&D laboratory of several thousands of square meters with advanced facilities in Caohejing Hi-Tech Park in Shanghai. The laboratory have 10 functional modules and more than 40 core technology platforms, led by a series of super-trillion phage display antibody libraries, and followed by a full range of essential R&D platforms for innovative antibody drug discovery, antibody engineering, in vitro and in vivo efficacy screening, pharmaceutical properties analysis, cell line construction, and process development. Sanyou Bio continues to provide new technologies, new products, services and solutions of "best quality, fastest speed and lowest cost". Sanyou Bio has established friendly business relationships with more than 100 pharmaceutical companies, drug development organizations and diagnostic product development companies around the world.
"Excellence and innovation, pursuit of dreams, striving and evolving, for the benefit of patients", holding these principles and beliefs, Sanyou Bio looks forward to working with our customers and partners to build a long-term synergistic growth ecosystem and a healthy society.
You're welcome to
send an email and tell us what's your need, and we will get back to you ASAP.
Sincerely.
Qingyang
Customer Specialist
Sanyou Biopharmaceuticals Co., Ltd.
Address: Building 6, No.188 Xinjunhuan Road,
Minhang District, Shanghai, China 201114
Email: service@sanyoubio.com
Tel: +86-21-3368 1627
Website: www.sanyoubio.com